-
วิทยาศาสตร์
-
มัธยมศึกษาตอนปลาย
-
ม.6 (มัธยมศึกษาปีที่ 6)
-
เคมี
-
English
คำแนะนำของผู้เขียน
Type of reaction: Free radical substitution reaction. It is a monosubstitution reaction because there is only one hydrogen atom in alkane is substituted by halogen free radical.
Step 1: Initiation step • In the present of uv light/heat, the covalent bond of Cl2 undergoes homolytic cleavage to produce free radicals. Function of uv light: provide energy for homolytic cleavage of Cl2
Step 2: Chain Propagation Step • Cl free radicals collide with methane to form HCl and methyl free radicals. Methyl free radicals react with Cl2 to form haloalkane and generate the chlorine free radicals
Step 3: Chain Termination Step • The reaction stops when the two free radicals collide and combine with one another
-
วิทยาศาสตร์
-
มัธยมศึกษาตอนปลาย
-
ม.6 (มัธยมศึกษาปีที่ 6)
-
เคมี
-
English
คำแนะนำของผู้เขียน
Type of reaction: Free radical substitution reaction. It is a monosubstitution reaction because there is only one hydrogen atom in alkane is substituted by halogen free radical.
Step 1: Initiation step • In the present of uv light/heat, the covalent bond of Cl2 undergoes homolytic cleavage to produce free radicals. Function of uv light: provide energy for homolytic cleavage of Cl2
Step 2: Chain Propagation Step • Cl free radicals collide with methane to form HCl and methyl free radicals. Methyl free radicals react with Cl2 to form haloalkane and generate the chlorine free radicals
Step 3: Chain Termination Step • The reaction stops when the two free radicals collide and combine with one another
สำรวจแผ่นงาน
Redoks (U- Tiub)
- Science
- 12th grade
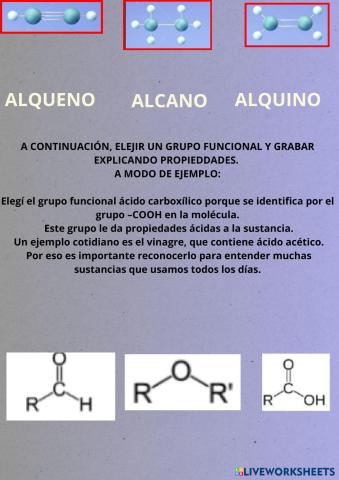
Grupos funcionales
- Science
- 12th grade
LEMBAR REFLEKSI
- Science
- 12th grade
 อยากได้แบบฝึกหัดฟรีนับพัน (ไม่มีสิ่งรบกวนเลย)?
อยากได้แบบฝึกหัดฟรีนับพัน (ไม่มีสิ่งรบกวนเลย)?