-
Chemistry
-
Chemistry
-
Age 17+
-
level: high school
-
English
Author's Instructions
What is a chemical formula?
Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elements hydrogen (H) and oxygen (O) and that it takes two atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), and oxygen (O). To make a molecule of this compound, you need one atom of sodium, one atom of nitrogen, and three atoms of oxygen.
How to write chemical formulas:
How do chemists know how many atoms of each element are needed to build a molecule? For ionic compounds, oxidation numbers are the key. An element’s oxidation number is the number of electrons it will gain or lose in a chemical reaction. We can use the periodic table to find the oxidation number for an element.
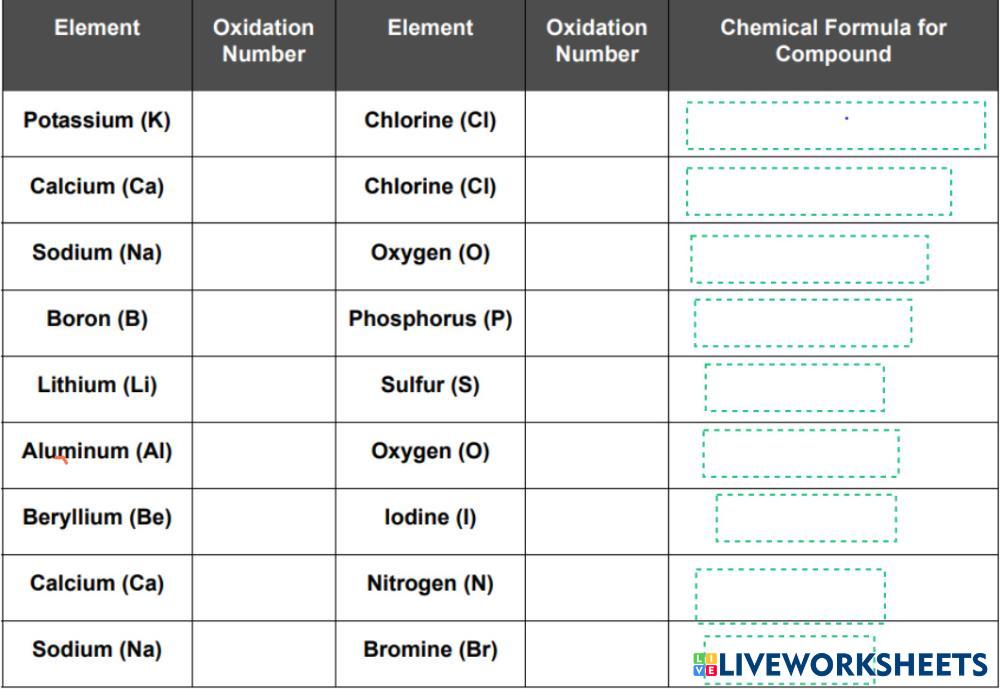
Directions: Use the periodic table to find the oxidation numbers of each element. Then write the correct chemical formula for the compound formed by the following elements:
-
Chemistry
-
Chemistry
-
Age 17+
-
level: high school
-
English
Author's Instructions
What is a chemical formula?
Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elements hydrogen (H) and oxygen (O) and that it takes two atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), and oxygen (O). To make a molecule of this compound, you need one atom of sodium, one atom of nitrogen, and three atoms of oxygen.
How to write chemical formulas:
How do chemists know how many atoms of each element are needed to build a molecule? For ionic compounds, oxidation numbers are the key. An element’s oxidation number is the number of electrons it will gain or lose in a chemical reaction. We can use the periodic table to find the oxidation number for an element.
Directions: Use the periodic table to find the oxidation numbers of each element. Then write the correct chemical formula for the compound formed by the following elements:
 📚 New Feature: Share worksheets & get automatic grading via Google Classroom 🎓
📚 New Feature: Share worksheets & get automatic grading via Google Classroom 🎓